FAQs
Food Allergy
What is food allergy?
What is a food allergy? (4 minute video)
Food allergies and eating out (5 minute video)
How to successfully prepare food free of a food allergen
Food Allergy or Food Intolerance?
For the millions of people with food allergies, there is good news regarding food labeling
Back to school Tool kit for school nurses
What is Eosinophilic Esophagitis (EoE)? (3 minute video)
Can food allergy play a role in acid reflux? (4 minute video)
Food allergies, asthma, and bullying (4 minute video)
What is anaphylaxis? (4 minute video)
Allergy
Fall Allergies
Diagnosing allergies – All about allergy testing
Ragweed can lead to fall misery for patients with allergies
Learn about allergies in children here
Pollen and mold counts
Outdoor allergens: Tips to Remember
Allergy shots: Tips to Remember
Allergy Overview
Managing pet allergies
Pet allergy Overview
More pet allergy information
Managing indoor allergen culprits
Gardening with seasonal allergies
Knowing allergy fact from fiction
Asthma
Infections
More Allergy and Asthma Information
COVID Vaccine
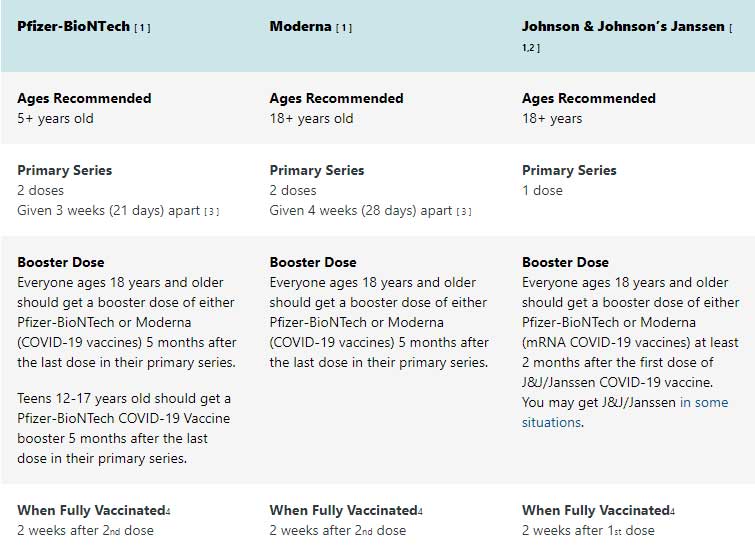
Who will get the vaccine?
Currently, all patients over age 5 are eligible to receive a COVID-19 vaccine.
- If you had a severe allergic reaction after a previous dose or if you have a known (diagnosed) allergy to a COVID-19 vaccine ingredient, you should not get that vaccine. If you have been instructed not to get one type of COVID-19 vaccine, you may still be able to get another type.
- CDC has updated its recommendations for COVID-19 vaccines with a preference for mRNA (Pfizer-BioNTech or Moderna) vaccines. Learn more about the updated guidance on the use of Janssen (Johnson & Johnson) COVID-19 vaccine.
- You should get your second shot as close to the recommended 3-week or 4-week interval as possible. You should not get the second dose early.
- As with vaccines for other diseases, people who are up to date on their COVID-19 vaccines are optimally protected. Learn more about staying up to date on your COVID-19 vaccines.
How do the Pfizer and Moderna mRNA vaccines work?
The vaccines contain synthetic mRNA, which is genetic information used to make the SARS-CoV-2 spike protein. The spike protein is the part of the virus that attaches to human cells. The spike protein alone cannot cause COVID-19. Once the spike protein is created it causes the immune system to make antibodies against the virus. These antibodies can provide protection if a person comes into contact with the virus. The mRNA vaccines are non-infectious and do not enter the human cell nucleus so they cannot be inserted into human DNA. Additionally, mRNA is rapidly broken down, and this theoretically reduces the chances of long-term side effects. The mRNA vaccines do not have the ability to cause cancer.
Will the COVID-19 vaccines be safe?
To date, no serious safety concerns have been reported by an independent data and safety monitoring board overseeing Phase 3 trials of the Pfizer and Moderna mRNA COVID-19 vaccines. Both vaccines met the safety requirements outlined by the FDA to seek EUA. In the safety analysis, patients were followed for 2 months after they received their second dose of the vaccine.
What is an Emergency Use Authorization (EUA)?
Emergency Use Authorization (EUA) occurs when the Food and Drug Administration (FDA) allows a drug or vaccine to be used during a public health emergency. The FDA may choose to grant EUA once studies have demonstrated the safety and effectiveness of a vaccine but before the manufacturer has submitted, or the FDA has completed its formal review of the license application. EUAs provide timely access to critical medical products during a medical emergency when there are no sufficient treatments or vaccines available.
Are the COVID-19 vaccines rigorously tested?
Yes. Clinical trials have evaluated potential COVID-19 vaccines in tens of thousands of study participants to generate the scientific data and other information needed by FDA to determine safety and effectiveness. Clinical trials are conducted according to the rigorous standards set forth by the FDA.
Why is vaccine development happening so fast?
The vaccine process is happening faster because vaccine research and development, clinical trials, manufacturing, and plans for distribution are occurring at the same time. This method removes delays that occur when these processes are carried out one after the other. Steps to ensure safety are not being eliminated.
If I get vaccinated, do I still need to wear a mask and social distance?
Yes. The CDC recommends following all safety recommendations such as masking and social distancing until there is a high enough vaccination rate that ensures we are closer to herd immunity and our policy changes.
Should I be vaccinated if I’ve already had COVID-19?
Yes. Although you may have a period of immunity after having COVID-19, a vaccine may offer more protection.
How many doses are needed and why?
Nearly all COVID-19 vaccines being studied in the United States require two injections. The first injection starts building protection, but everyone has to come back a few weeks later for the second one to get the most protection the vaccine can offer.
For 2 dose vaccines, what happens if I only receive one dose of the vaccine and not both?
It is recommended to receive both doses of the vaccine. If only one vaccine is received, immunity cannot be guaranteed.
What side effects will the vaccine have? Are there going to be long-term side effects?
In Phase 3 clinical trials, the most common side effects reported were as follows:
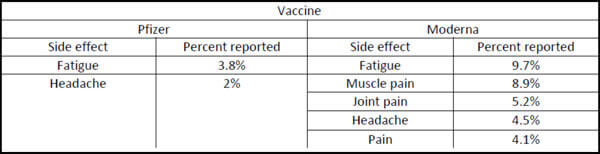
Side effects have been reported to be short-lived and happen within the first few days of receiving the vaccine. Side effect occurrence is typically higher after the second dose of vaccine. Historically, long-term side effects from vaccines have been rare.
Why is the development of a vaccine critical to controlling COVID-19?
Stopping a pandemic requires using all the tools available. Vaccines work with your immune system so your body will be ready to fight the virus if you are exposed. Other steps, like covering your mouth and nose with a mask and staying at least 6 feet away from others, help reduce your chance of being exposed to the virus or spreading it to others. Together, COVID-19
vaccination and following CDC’s recommendations to protect yourself and others will offer the best protection from COVID-19.
Can I get COVID-19 after receiving the vaccine?
No vaccine is 100% effective at preventing infection. The two vaccines that have been submitted to FDA for emergency use authorization have been reported to be over 90% effective. An effective vaccine will lower your risk of getting the infection and will also lower your risk of severe disease if you are infected. A goal of a COVID-19 vaccine is also to make it less likely that
COVID- 19 can spread to others. Once clinical trials are complete and the results are reviewed, FDA and CDC will be able to share more specific details.
Visit the CDC website to learn more about vaccine safety standards: https://www.cdc.gov/vaccines/covid-19/
If you develop COVID-19 symptoms after getting the vaccine should you quarantine?
Yes. It typically takes a few weeks for the body to build immunity after vaccination. That means it is possible a person could be infected with the virus that causes COVID-19 just before or just after vaccination and gets sick. This is because the vaccine has not had enough time to provide protection. If you have COVID-19 virus symptoms after getting the vaccine or at any time, you should contact your health care provider and consider getting tested for COVID-19.
How long will immunity last after I get vaccinated? Will I need to be vaccinated every year?
The length of immunity following vaccination is not yet known for COVID-19. Given the novel nature of this virus and vaccine development, long-term data is not yet available to guide future vaccine protocols. You can visit the CDC website to learn more about vaccine safety standards: https://www.cdc.gov/vaccines/covid-19/
